Yoga for Parkinson's Disease Part II: Pathology, Risk Factors, and General Treatment Strategies
- Jackie Allen
- Mar 3, 2023
- 12 min read

Hey hey readers! I am so glad you are here! In this 5-part blog series, I am reviewing research that has examined yoga, and other forms of physical activity (PA), in individuals with Parkinson’s Disease (PD). In Part I, I went over what PD is and what the common symptoms are. If you have not read that post yet, it might be helpful to check that one out before reading this post (link here).
In this installment, I aim to describe the pathology, risk factors, possible preventions, and general treatment strategies for PD. Stay tuned for part III which will seek to review what the science seems to suggest about yoga’s benefit for PD symptomology. Part IV will discuss other forms of PA and the effects therein on the motor and nonmotor symptoms of PD. My neuroscience heart is super excited for Part V of this series, in which I will go over the neurobiology of the brain areas involved in PD! As I have begun to work with more and more people with Parkinson's disease (PWPD) in my speech therapy field, I have been feeling this growing desire to pull out my old neuroscience notes from grad school in order to connect what I do in speech therapy with PWPD, with my yoga mind and heart, to my understanding of the brain and nervous system. So, this series may be a little neuroscience-hevy, but who doesn't love cool neuro facts?

As discussed in Part I, the rates of PD seem to be increasing. The disease itself affects motor and nonmotor functioning alike. Motor symptoms are wide-ranging and individual-specific, but typically PWPD experience some or all of the following: muscle tightness (rigidity), difficulties with posture (postural instability), slowed movement (bradykinesia), resting tremor, trouble with balance, difficulty walking, and an increased risk of falling. Nonmotor symptoms are also highly variable, but often include: depression, anxiety, cognitive difficulty (memory, problem solving, planning, etc.), chronic pain, autonomic system dysfunction (heart rate, blood pressure, etc.), sleep dysfunction, and/or loss of smell (hyposmia).
Let’s chat about the pathology (aka biological explanation), risk factors, and general treatment strategies.
PATHOLOGY
Most cases of PD are idiopathic, meaning the cause for why any one person gets it is unknown. There are some cases of PD that are inherited (known as familial), but these are more rare, accounting for about 10% of PD cases worldwide. Familial cases of PD are typically due to a mutation in genes such as PARKIN, DJ-1, PINK1, and APT13A2. Even though the exact causes of idiopathic (or sporadic) PD are not known, the etiology, or underpinnings, of the disease process itself is relatively well understood. What isn’t well understood, though, is the mechanisms that drive the spread and progression of the pathology, where it gets worse over time.
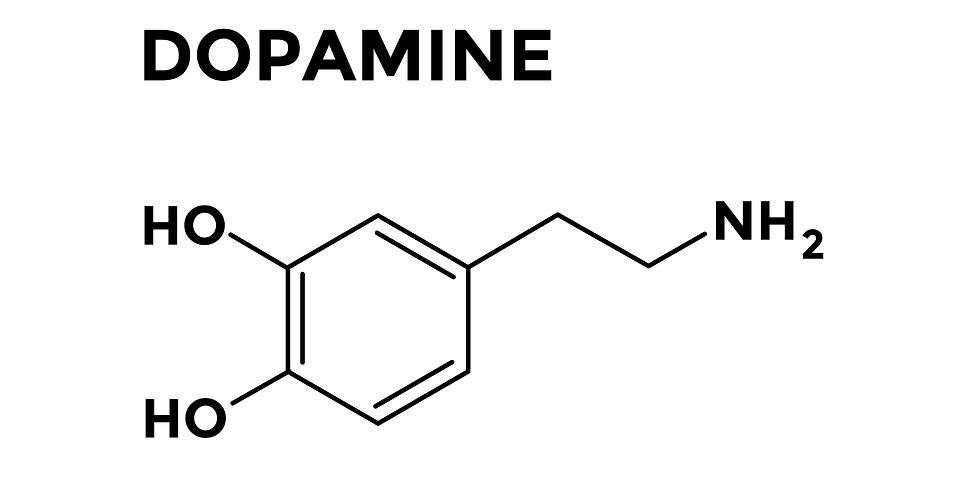
In its simplest terms, the pathology of PD results from a progressive loss of neurons that produce dopamine (DA) in a part of the brain known as the substantia nigra pars compacta (SNpc). The SNpc is part of a larger brain area, known as the basal ganglia (BG), which is sort of tucked into the center, bottom-ish part of the brain (i.e. the midbrain), with one in either hemisphere (i.e. left and right sides of the brain). DA is a very important neurotransmitter (aka chemical messenger in the brain) produced by dopaminergic (DAergic) neurons. DA is involved in regulating things such as movement, memory, learning, motivation, and appetite/feeding behaviors. As the DAergic neurons progressively die off in the SNpc, the feedback loops in the BG cannot operate properly because the essential input from these neurons is gone (or diminished). More details about DA and the BG will be reviewed in Part V of this series, where I will review the neuroanatomy and physiology of the brain areas involved in PD (eek! So excited to go back to my old neuroscience-ey roots). The image below depicts the location of where the DAergic neurons die off in the SNpc of the BG.

In PD, insoluble aggregates (aka clumps) and fibrils (aka microscopic fibers) begin to deposit and accumulate between, and within, the DAergic neurons. These clumps and fibrils are known as Lewy bodies (LB) and Lewy Neurites (LN), respectively, and consist mainly of a protein called alpha-synuclein. The presence of LBs and LNs disprupts cell-to-cell, and within-cell, communication, and ultimately contributes to the cell death of DAergic neurons. LBs are represented by the orange globs in the image below.

Although the main causes of sporadic PD are not fully understood or known, the predominant theories suggest that PD might be caused by oxidative stress, neuroinflammation, mitochondrial dysfunction, and environmental factors like drugs and pesticides. Oxidative stress is defined as a disturbance in the balance between free radicals (aka reactive oxygen species – ROS) and antioxidant defenses. Free radicals are very damaging to cell structures and components because they are so reactive. Antioxidants help to neutralize them so they cannot cause damage to cellular components. Oxidative stress is actually thought to play a key role in the progressive loss of DAergic neurons in the SNpc. Oxidative stress can cause inflammation in the neurons, impacting their function and ultimately causing their death. Mitochrondria are cell organelles that work to produce energy for the cell; they can become disrupted by a variety of factors, including free radicals, where entire cellular metabolism can be altered. Pesticides and certain drugs/chemicals can also cause oxidative stress and inflammation to the brain. These cellular events theoretically lead to the loss of DAergic neurons in the SNpc as well as the accumulation of the LBs and LNs in that same brain area.
RISK FACTORS AND PREVENTION
Although there is no clear consensus on the exact risk factors and prevention strategies for PD, there are some ideas that have been put forth in the science world.
Potential risk factors for developing PD are various and include:
Exposure to pesticides – pesticides can cause oxidative stress and disrupt mitochondria function
Brain injury – Traumatic Brain Injury (TBI) and concussions (especially if repeated) can disrupt the blood brain barrier (BBB – the protective barrier between the physiological landscape of the brain and the environment of the body), impair mitochondria function, and lead to accumulation of alpha-synuclein (the main protein of LBs and LNs – recall the clumps and fibers that congregate around DAergic neurons in PD causing cell death)
Weight – some studies suggest that being overweight or obese will bring a higher risk for developing PD
Dairy consumption – there is a positive correlation between PD and consuming dairy
Potential preventative strategies have limited or conflicting evidence and include:
Certain beverage consumption – interestingly, people who regularly drink alcoholic, coffee, and black tea have a lower risk for PD than people who do not drink those beverages.
Use of antihypertensive drugs – this seems to be associated with a reduced risk for PD
Physical activity – associated with lower risk for PD and reduced symptomology (more in Parts III & IV)
Healthy diet – can reduce the risk and symptom severity of PD
GENERAL TREATMENT STRATEGIES
The main purpose of any treatment for PD is to slow down the progression of the disease, reduce the clinical symptoms, and improve QOL. There is no complete cure for PD at this time, but rather, the treatments described below attempt to mitigate the symptoms of PD to some extent.
Pharmacology/drugs
PD is generally treated with drugs in an effort to control the motor and nonmotor symptoms.
Drugs for the motor symptoms include DAergic medicines and non-DAergic medicines. DAergic drugs aim to pharmacologically replace DA in the brain. Levodopa (aka L-DOPA) is the most common and efficient medication for the motor symptoms of PD. L-DOPA is a precursor molecule for DA, and it gets converted to DA by a special enzyme naturally occuring in the brain called DOPA decarboxylase. While L-DOPA is usually well-tolerated and offers a robust and stable alleviation of PD symptoms, at least initially (aka “honeymoon phase”), there is a 40% chance of developing motor complications (other muscle abnormalities known as dyskinesia) after several years of use. Also, L-DOPA can wear-off over time and not be as effective, requiring some PWPD to cycle on/off L-DOPA. DAergic medicines aren’t without side effects either. Common side effects from DAergic therapies include hallucinations, hypotension (abnormally low blood pressure), nausea, vomiting, fatigue, pathological gambling, compulsive shopping, and hypersexuality.

Non-DAergic drugs for motor symptoms include anticholinergics, which essentially help to correct the imbalance between DA and another neurotransmitter called acetylcholine (Ach), which can help control tremors. There is also an antiviral medication, known as Amantadine, which relieves motor symptoms as well. Finally, cannabis has also been studied, especially recently, and it appears that cannabis can quite significantly alleviate tremors, bradykinesia (slow movement), AND rigidity.
There are also drug treatments for the nonmotor symptoms. Chronic pain (discussed in Part I), which is common in PD, can be treated with NSAIDs (e.g. advil), opioids (if pain is bad enough), SSRIs (selective serotonin reuptake inhibitors), anticonvulsants, and even antidepressants. Medicines can also be prescribed for managing anxiety and depression, but this gets very complicated for PWPD. Specifically, potential drug interactions exist between antidepressants and other PD medicines, making finding the right balance of medicines way more challenging for physicians and patients alike. Also, some serotonergic drugs (drugs for replacing serotonin) can exacerbate PD symptoms.
In general, while pharmacological interventions definitely help, especially the main motor symptoms, these treatments are often insufficient for improving nonmotor symptoms, as well as balance control, posture deficits, and walking difficulties. There also is currently no fully proven pharmacology therapy that can modify or slow the disease progression.
Neuroprotective treatments
Neuroprotective treatments attempt to block the disease process or underlying pathogenesis. Evidence is limited, but includes:
Glutathione - this chemical is the primary antioxidant in the brain, so it can potentially help reduce oxidative damage from free radicals
Drinking green tea - this has been shown to potentially reverse neurodegeneration in PD as well as Alzheimer's disease (which also is associated with accumulation of LBs)
Nicotine - interestingly, this is considered beneficial for PD, as some of nicotine’s derivatives decrease oxidative stress and neuroinflammation as well as improve DAergic neuron survival
Coenzyme Q10 - this is another common antioxidant that can enhance mitochondrial function, potentially reversing the effects of mitochrondia dysfunction

Rehabilitation
Rehabilitative therapy, including occupational, physical, and speech therapy, uses various neuromuscular, myofascial, cognitive, and linguistic approaches to try to slow the disease process and prevent further decline as well as maintaining function over time. Each of these therapies can occur in a hospital, assisted living facility, outpatient setting, or private practice. Insurance typically pays or reimburses for these services, if deemed medically necessary by therapist and physician. This particular treatment approach is near and dear to my heart because I am a speech therapist. I have worked quite a bit with PWPD, and I also studied the DAergic system for years in my first Master’s degree. Thus, this is a population that I feel very connected to. While each patient I work with is unique and special, in general, I work on the following areas in therapy: respiratory support (increasing strength, endurance, coordination, and agility of breathing muscles to support ADLs); swallowing skills (chewing, swallowing, etc.); voice, (loudness, pitch, quality); articulation (clarity of words spoken); cognitive skills (memory, attention, problem solving, etc.); and language (expression and comprehension). I generally program my therapy frequency at 2-4x per week, depending on the patient, where the session length is typically 45min-1 hour. The fields of occupational, physical, and speech therapy are clinical, medical fields that are evidence-based and driven by science, just like any other medical field.

Alternative and complementary treatment
While pharmacological interventions can be pretty helpful for the motor symptoms in PD, the nonmotor features of PD (e.g. cognitive, sleep, autonomic dysfunction, etc.) are often progressive and may not respond to drugs, having a profound impact on a person’s QOL. Thus, many PWPD pursue complementary therapies.
Alternative and complementary therapies include:
Mind-body therapies – e.g. yoga, Tai Chi, Qi Gong, etc.
Resistance training - strength training, weight-lifting
Proprioceptive training and conventional balance exercise – training balance and the ability to sense where the body is in space
Aerobic exercise – walking, running, etc.
Dance – dancing to rhythmic music
Massage and acupuncture
Obviously, there are many alternative therapies that exist, and WAY more detail will be provided in Parts III & IV of this series, where I will dig into the research for yoga specifically and physical activity in general in PD, so stay tuned!

Other Forms of Treatment
There are surgical, gene, and cell replacement therapies for PD; however, these treatments are out of my scope of knowledge to discuss in great detail. There is also a treatment known as deep brain stimulation (DBS), where electrodes stimulate the parts of the brain affected by PD. DBS has been shown to reduce chronic pain, tremor, bradykinesia, and rigidity. The evidence for DBS for improving posture and gait is limited, though.
SUMMARY
My goodness thank you so much for staying with me until the end! I know that was a lot of information to take in. PD results from a loss of DAergic neurons in the substantia nigra pars compacts (SNpc) of the basal ganglia, which is located in the midbrain. The loss of these neurons seems to occur partly due to accumulation of aggregates and fibers called lewy bodies and lewy neurites, respectively. Oxidative stress and mitochondrial damage also seem to contribute to the death of DAergic neurons in the SNpc. While most PD cases are idiopathic, meaning of unknown origin, there are some identified risk factors, including head injuries, pesticides and environmental toxins, and maybe even dairy consumption. There are several lines of treatment, including pharmacology (L-DOPA being the most commonly prescribed drug), rehabilitative therapy (physical, occupational, and speech therapy), neuroprotective treatments (antioxidants, green tea, nicotine) and alternative treatments (yoga, aerobic exercise, dance, strength training, massage, etc.). Pharmacological treatment typically aims to treat motor symptoms, although there are drug options to help manage the nonmotor symptoms (such as chronic pain, anxiety, etc.). Alternative treatments, like yoga, can help both the motor and nonmotor symptoms, but this will be discussed in waaaayyy greater detail in Parts III and IV of this series. Currently there is no cure or treatment that stops the ultimate progression of the disease. Thank you so much for reading this post. Hopefully you will stick me for this entire series. As a devout yoga practitioner and yoga teacher, I personally have seen how transformative and healing yoga can be. I cannot wait to share this research with you all next month in Part III (spoiler alert - yoga helps mitigate the symptoms of PD). I absolutely love the pursuit of knowledge, especially when that pursuit may help to improve the lives of other beings. So thank you truly from the bottom of my yogi heart for joining me on this journey to better understand, and hopefully help, Parkinson's disease. See you next month!
As always, the information presented in this blog post is derived from my own study of human movement, anatomy and physiology, yoga, and neuroscience. If you have questions about Parkinson's disease specific for you, please follow up with your physician, neurologist, or physical therapist. If you are interested in private yoga and/or personal training sessions with me, Jackie, email me at info@lotusyogisbyjackie.com for more information about my services. Also, please subscribe to my website so you can receive my monthly newsletters (scroll to the bottom of the page where you can submit your email address). This will help keep you "in-the-know" about my latest blog releases and other helpful yoga and wellness information. Thanks for reading!
~Namaste, Jackie Allen, M.S., M.Ed., CCC-SLP, RYT-200, RCYT, NASM-CPT, NASM-CES, NASM-CNC, NASM-SFC, NASM-WLS
References:
Bhalsing, K.S. et al. (2018). Role of Physical Activity in Parkinson's Disease. Annals of Indian Academy of Neurology. 21(4): 242 - 249.
Ban, M. et al. (2021). The Effects of Yoga on Patients with Parkinson's Disease: A Meta-Analysis of Randomized Controlled Trials. Behavioral Neurology. 2021: 1 - 11.
Cheung, C. et al. (2018). Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson's disease: a pilot randomized controlled trial. Pilot and Feasibility Studies. 4: 162 - 173.
Crossman, A.R. & Neary, D. (2010). Neuroanatomy: An Illustrated Colour Text. Elsevier Limited.
Deuel, L.M. & Seeberger, L.C. (2020). Complementary Therapies in Parkinson Disease: a Review of Acupuncture, Tai Chi, Qi Gong, Yoga, and Cannabis. Neurotherapeutics. 17: 1434 - 1455.
Dong, J., et al. (2016). Current Pharmaceutical Treatments and Alternative Therapies of Parkinson's Disease. Current Neuropharmacology. 14: 339 - 355.
Edinoff, A. et al. (2020). Chronic Pain Treatment Strategies in Parkinson's Disease. Neurology International. 12: 61 - 76.
Elangovan, N. et al. (2020). Hatha yoga improves standing balance but not gait in Parkinson's disease. Sports Medicine and Health Science. 2(2020): 80 - 88.
Fan, B. et al. (2020). What and How Can Physical Activity Prevention Function on Parkinson's Disease? Oxidative Medicine and Cellular Longevity. 1 - 12.
Hao, Z. et al. (2022). Effects of Ten Different Exercise Interventions on Motor Function in Parkinson's Disease Patients - A Network Meta-Analysis of Randomized Controlled Trials. Brain Sciences. 12: 1 - 25.
Iverson, L.L. et al. (2010). Dopamine Handbook. Oxford University Press, Inc. New York, NY.
Jin, X. et al. (2020). The Impact of Mind-Body Exercises on Motor Function, Depressive Symptoms, and Quality of Life in Parkinson's Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 17: 1 - 16.
Khuzema, A. et al. (2020). Effect of home-based Tai Chi, Yoga, or conventional balance exercise on functional balance and mobility among persons with idiopathic Parkinson's disease: An experimental study. Hong Kong Physiotherapy Journal. 40(1): 39 - 49.
Kwok, J.Y.Y. et al. (2019). Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People with Parkinson Disease- A Randomized Clinical Trial. JAMA Neurology. 76(7): 755 – 763.
Kwok, J.Y.Y., et al. (2017). The effects of yoga versus stretching and resistance training exercises on psychological distress for people with mild-to-moderate Parkinson's disease: study protocol for a randomized control trial. Trials. 18: 1 - 13.
Myers, P.S. et al. (2020). Yoga improves balance and low back pain, but not anxiety, in people with Parkinson's disease. International Journal of Yoga Therapy. 30(1): 41 - 48.
Puymbroeck, M.V. et al. (2018). Functional Improvement in Parkinson's Disease Following a Randomized Trial of Yoga. Evidence-Based Complimentary and Alternative Medicine. 2018: 1 - 8.
Sharma, N.K. et al. (2015). A randomized controlled pilot study of the therapeutic effects of yoga in people with Parkinson's disease. International Journal of Yoga. 8: 74 - 79.
Wu, C. et al. (2021). Effects of Aerobic Exercise and Mind-Body Exercise in Parkinson's Disease: A Mixed Treatment Comparison Analysis. Frontiers in Aging Neuroscience. 13: 1 - 10.
Comments